


Take 2 capsules daily.
Not to be taken by transplant patients or patients taking immunosuppressing drugs. Consult a physician prior to use if you are pregnant, nursing, taking medication or if you have a medical condition.
KEEP OUT OF REACH OF CHILDREN

Other Ingredients: Magnesium Stearate (Vegetable), Vegetable Capsule (Hypromellose, Red Iron Oxide (Color)).
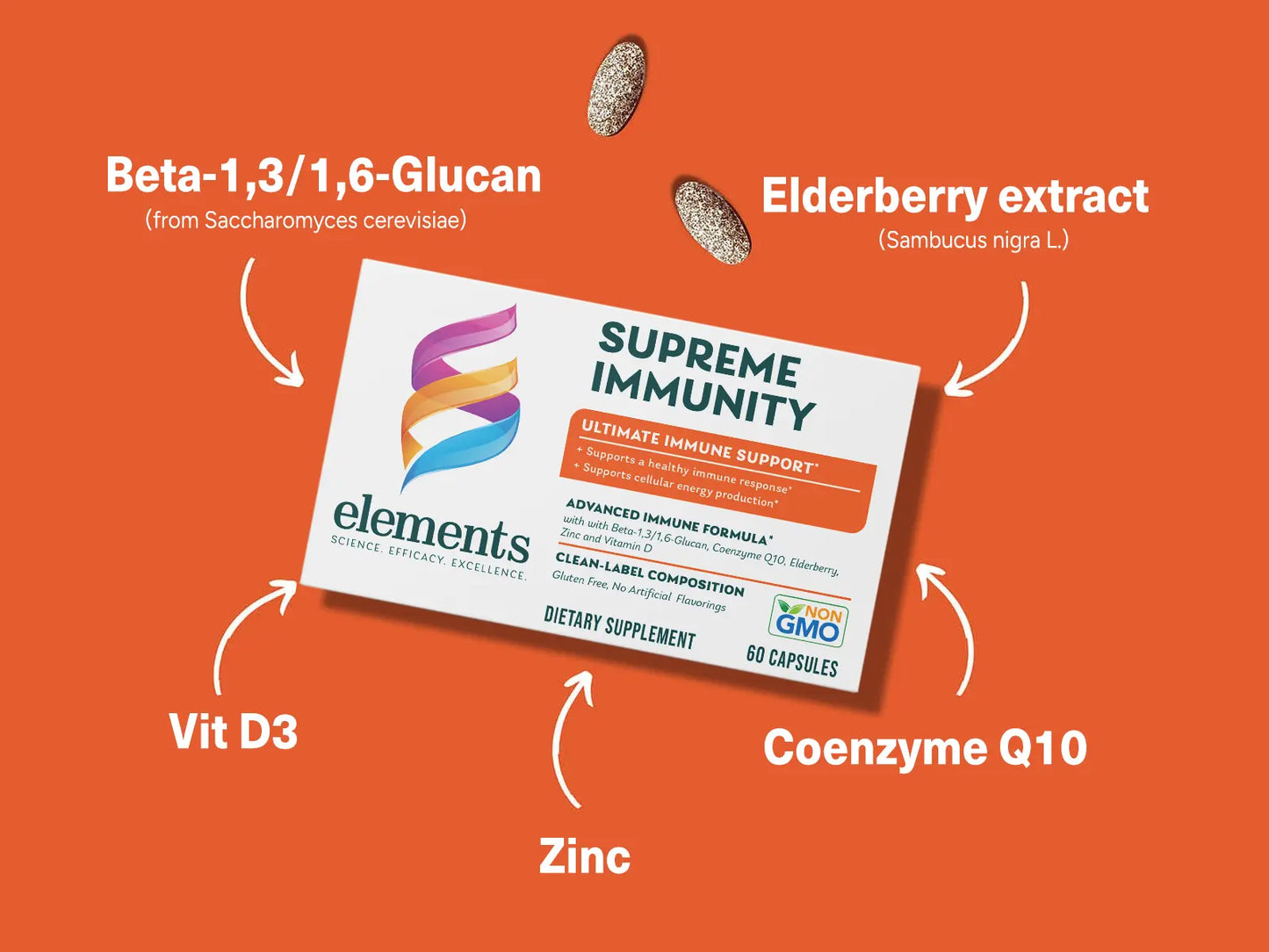
Advanced Immune Formula
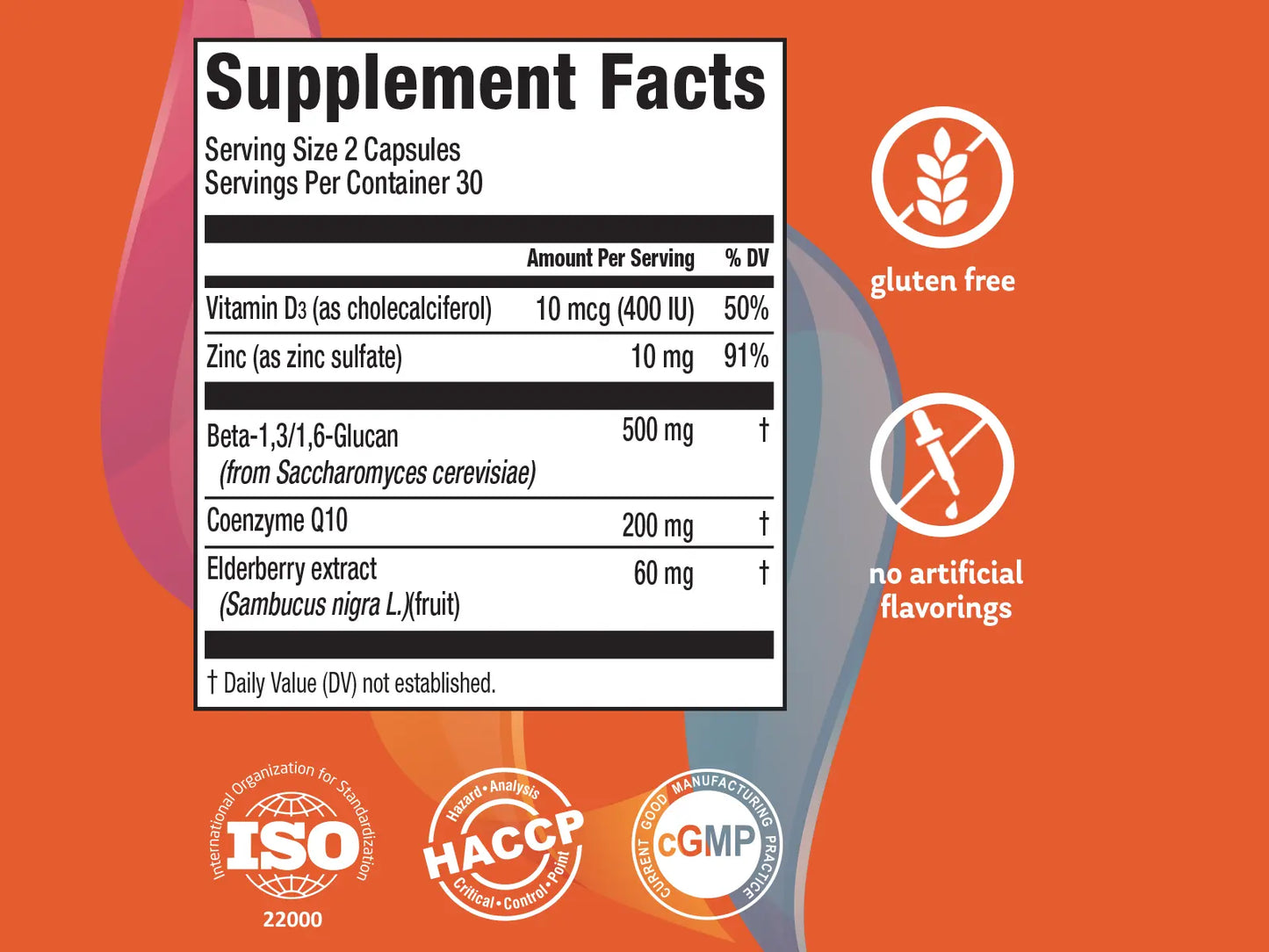
Supreme Immunity formula has been blended with unique selection of ingredients along with vit. D and zinc which provide all year-round immune support and wellbeing.* Our everyday immune formula is enhanced with BETA-1,3/1,6-GLUCAN and ELDERBERRY EXTRACT to support a healthy immune function.* Supreme Immunity also has COENZYME 10 to support cellular energy production.*
-

VITAMIN D
Vitamin D is an essential nutrient needed to keep bones, muscles and teeth healthy.* Though there are natural sources of vitamin D, they are limited only to a small number of foods. Our body creates vitamin D from cholesterol in the skin only when it’s exposed to the direct sunlight. That is why it is important to maintain the levels of vitamin D through additional supplementation. It also contributes to the normal function of the immune system.* -

ZINC
Zinc is an essential mineral that plays vital role in our body.* The role of Zinc is crucial for the normal growth, physical development, nutrient metabolism, cell function and division, DNA synthesis and protection of cells from oxidative stress. It also contributes to the normal function of the prostate.* -

BETA-1,3/1,6-GLUCAN
Beta-1,3/1,6-Glucan is a carbohydrate derived from the cell wall of Saccharomyces cerevisiae, commonly known as Bakers’ yeast. According to scientific studies, Beta-1,3/1,6-Glucan supports immune system through its ability to maintain several aspects of the body’s healthy immune function.*
-

COENZYME Q10
Coenzyme Q10 is fat soluble substance, naturally produced in our body, which plays an important role in cellular energy production.* Every human cell has it in its mitochondria, known as power houses of the cell. Coenzyme Q10 is a powerful antioxidant which protects cells from oxidative stress.* Highest concentrations of Coenzyme Q10 could be found in our heart, lungs, liver and kidneys.
-

ELDERBERRY EXTRACT
Elderberry extract from Sambucus nigra L. which has bioactive substances, such as flavonoids, phenolic acids, anthocyanins and polysaccharides. Sambucus nigra is also a potent antioxidant, supporting a healthy immune function.*
VITAMIN D
ZINC
BETA-1,3/1,6-GLUCAN
Beta-1,3/1,6-Glucan is a carbohydrate derived from the cell wall of Saccharomyces cerevisiae, commonly known as Bakers’ yeast. According to scientific studies, Beta-1,3/1,6-Glucan supports immune system through its ability to maintain several aspects of the body’s healthy immune function.*
COENZYME Q10
Coenzyme Q10 is fat soluble substance, naturally produced in our body, which plays an important role in cellular energy production.* Every human cell has it in its mitochondria, known as power houses of the cell. Coenzyme Q10 is a powerful antioxidant which protects cells from oxidative stress.* Highest concentrations of Coenzyme Q10 could be found in our heart, lungs, liver and kidneys.
ELDERBERRY EXTRACT
Elderberry extract from Sambucus nigra L. which has bioactive substances, such as flavonoids, phenolic acids, anthocyanins and polysaccharides. Sambucus nigra is also a potent antioxidant, supporting a healthy immune function.*
- EFSA Panel on Dietetic Products N and A (NDA). EFSA J. 2009;7(9):1229-n/a. doi:10.2903/j.efsa.2009.1229
- EFSA Panel on Dietetic Products N and A (NDA). EFSA J. 2013;11(7):1-11. doi:10.2903/j.efsa.2010.1468
- Cesarone MR, Belcaro G, Di Renzo A, et al. Clin Appl Thromb. 2007;13(2):130-136. doi:10.1177/1076029606295957
- Patiroglu T, Kondolot M. Clin Respir J. 2013;7(1):21-26. doi:10.1111/j.1752-699X.2011.00268.x
- Moyad MA, Robinson LE, Zawada ET, et al. J Altern Complement Med. 2010;16(2):213-218. doi:10.1089/act.2010.16407
- Jensen GS, Patterson KM, Barnes J, et al. Open Nutr J. 2008;2:68-75. doi:10.2174/1874288200802010068
- Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Pediatrics. 2009;124(2):e172-e179. doi:10.1542/peds.2008-2666
- West NP, Horn PL, Pyne DB, et al. Clin Nutr. 2014;33(4):581-587. doi:10.1016/j.clnu.2013.10.002
- Di Pierro F, Colombo M, Zanvit A, Risso P, Rottoli AS. Drug Healthc Patient Saf. 2014;6:15-20. doi:10.2147/DHPS.S59665
- Vetvicka V, Richter J, Svozil V, Dobiášová LR, Král V. 2013;1(3):3-7. doi:10.3978/j.issn.2305-5839.2013.07.01
- Moyad MA, Robinson LE, Zawada ETJ, et al. Urol Nurs. 2008;28(1):50-55.
- Morales D, Rutckeviski R, Villalva M, et al. Carbohydr Polym. 2020;229:115521. doi:https://doi.org/10.1016/j.carbpol.2019.115521
- Dai X, Stanilka JM, Rowe CA, et al. J Am Coll Nutr. 2015;34(6):478-487. doi:10.1080/07315724.2014.950391
- Zembron-Lacny A, Gajewski M, Naczk M, Siatkowski I. J Physiol Pharmacol an Off J Polish Physiol Soc. 2013;64(2):249-254.
- Gaullier J-M, Sleboda J, Ofjord ES, et al. Int J Med Mushrooms. 2011;13(4):319-326.
- Spierings ELH, Fujii H, Sun B, Walshe T. J Nutr Sci Vitaminol (Tokyo). 2007;53(6):536-539. doi:10.3177/jnsv.53.536
- Bagwe S, Tharappel LJP, Kaur G, Buttar HS. J Complement Integr Med. 2015;12(3):175-185. doi:10.1515/jcim-2014-0039
- Crooks C V, Wall CR, Cross ML, Rutherfurd-Markwick KJ. Int J Sport Nutr Exerc Metab. 2006;16(1):47-64.
- Tojo R, Suárez A, Clemente MG, et al. World J Gastroenterol. 2014;20(41):15163-15176. doi:10.3748/wjg.v20.i41.15163
- AAVV. Fao Who. 2001. doi:10.1201/9781420009613.ch16
- Sanders ME, Merenstein D, Merrifield CA, Hutkins R. Nutr Bull. 2018;43(3):212-225. doi:10.1111/nbu.12334
- Dierksen KP, Moore CJ, Inglis M, Wescombe PA, Tagg JR. FEMS Microbiol Ecol. 2007;59(3):584-591. doi:10.1111/j.1574-6941.2006.00228.x
- Wescombe PA, Upton M, Dierksen KP, et al. Appl Environ Microbiol. 2006;72(2):1459-1466. doi:10.1128/AEM.72.2.1459-1466.2006
- Tagg JR. Indian J Med Res. 2004;119 Suppl:13-16.
- Gibson GR, Hutkins R, Sanders ME, et al. Nat Rev Gastroenterol Hepatol. 2017;14(8):491-502. doi:10.1038/nrgastro.2017.75
- He C-SS, Handzlik M, Fraser WD, et al. Exerc Immunol Rev. 2013;19(0):86-101.
- Wang T-T, Nestel FP, Bourdeau V, et al. J Immunol. 2004;173(5):2909-2912.
- He C-S, Fraser WD, Tang J, et al. J Sports Sci. 2016;34(1):67-74. doi:10.1080/02640414.2015.1033642
- Aranow C. Vol 59.; 2011. doi:10.2310/JIM.0b013e31821b8755
- FAO/WHO. Vol 85.; 2006. doi:10.1201/9781420009613.ch16
- Dardenne M. Eur J Clin Nutr. 2002;56 Suppl 3:S20-3. doi:10.1038/sj.ejcn.1601479
- Crooks C, Cross ML, Wall C, Ali A. Int J Sport Nutr Exerc Metab. 2010;20(3):224-235. doi:10.1123/ijsnem.20.3.224
- Thaiss CA, Zmora N, Levy M, Elinav E. Nature. 2016;535(7610):65-74. doi:10.1038/nature18847
- Jensen GS, Redman KA, Benson KF, et al. J Med Food. 2011;14(9):1002-1010. doi:10.1089/jmf.2010.0174
- Kupfahl C, Geginat G, Hof H. Int Immunopharmacol. 2006;6(4):686-696. doi:10.1016/j.intimp.2005.10.008
- Actor JK, Hwang S-A, Kruzel ML. Curr Pharm Des. 2009;15(17):1956-1973.
- Berlutti F, Pantanella F, Natalizi T, et al. Molecules. 2011;16(8):6992-7012. doi:10.3390/molecules16086992
- Gerasimov S V, Ivantsiv VA, Bobryk LM, et al. Eur J Clin Nutr. 2016;70(4):463-469. doi:10.1038/ejcn.2015.171
- Wheeler JG, Shema SJ, Bogle ML, et al. Ann allergy, asthma Immunol Off Publ Am Coll Allergy, Asthma, Immunol. 1997;79(3):229-233. doi:10.1016/S1081-1206(10)63007-4
- Meng H, Lee Y, Ba Z, et al. Mol Nutr Food Res. 2016;60(5):1161-1171. doi:10.1002/mnfr.201500665
- Taipale TJ, Pienihäkkinen K, Isolauri E, Jokela JT, Söderling EM. Pediatr Res. 2016;79(1-1):65-69. doi:10.1038/pr.2015.174
- Kaci G, Goudercourt D, Dennin V, et al. Appl Environ Microbiol. 2014;80(3):928-934. doi:10.1128/AEM.03133-13
- Gibson GR, Roberfroid MB. J Nutr. 1995;125(6):1401-1412. doi:10.1093/jn/125.6.1401
- Prietl B, Treiber G, Pieber TR, Amrein K. Nutrients. 2013;5(7):2502-2521. doi:10.3390/nu5072502
- Kamen DL, Tangpricha V. J Mol Med (Berl). 2010;88(5):441-450. doi:10.1007/s00109-010-0590-9
- Winchurch RA, Togo J, Adler WH. Clin Immunol Immunopathol. 1988;49(2):215-222.
- Wessels I, Maywald M, Rink L. Nutr . 2017;9(12). doi:10.3390/nu9121286
- Embria educational material
* These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.
What Our Customers Are Saying
Clean Label Composition
Our labels are clean, transparent, and exceed industry requirements.
-

Gluten Free
-

No Artificial Flavoring
-

Science Backed

















